Welcome to Your Exclusive Access
We are delighted
to welcome you to this exclusive page, accessible only through the unique URL shared with you following your recent conversation with Michaël Jeanty at the A3P event.
This page offers you the opportunity to schedule a personalized demo of our latest innovations and solutions, designed to meet the evolving needs of the biopharmaceutical industry.
We look forward to showcasing how our offerings can support your business and growth.

We Design and Produce Equipment for the Biopharmaceutical Industry
Becarv designs and manufactures patented equipment tailored to meet the specific needs of the biopharmaceutical industry. We focus on maintaining aseptic conditions, ensuring grade A continuity, and advancing single-use technologies, all the while complying with rigorous quality standards.
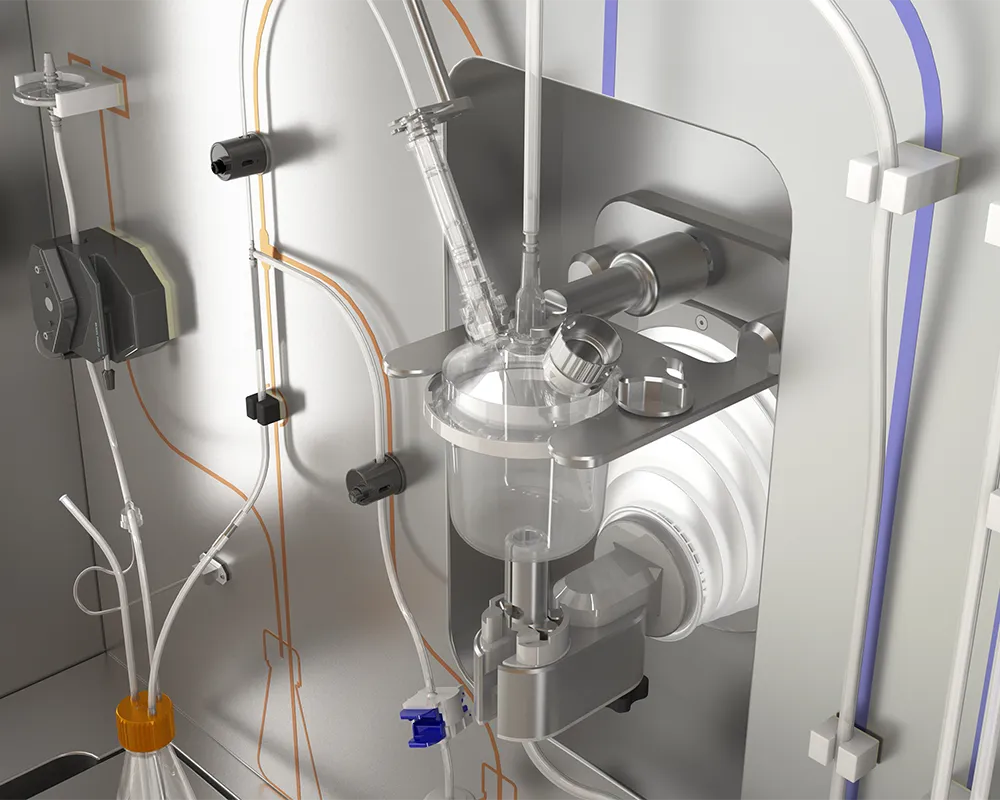
Closed Seeding Unit
Introducing our Closed Seeding Unit – a cutting-edge solution meticulously engineered to elevate the efficiency of cell line inoculation.
This automated, closed system streamlines workflows, enhancing productivity and repeatability in cell seeding through robotic operation, while minimizing contamination risks by ensuring the operator never directly contacts the product. Its digitalization enables seamless monitoring and provides full visibility into the production process. Additionally, it eliminates the need for inoculation rooms and isolators, significantly reducing capital expenditures for facility build-outs and upgrades, while lowering operational expenses by aligning the cost structure with production.
Modular Air Supply Solutions
Our modular equipment is designed to fit within a standard layout grid, enabling seamless integration into various facility designs and supporting scalable, flexible manufacturing processes. To accommodate diverse operational needs, our units are available in several sizes, ensuring an ideal fit for your specific spatial and process requirements.
Each environment can be equipped with either Fan Filter Units (FFU) or Filter Units (FU). Additionally, Becarv has developed a patented joint system that seals the container to the highest level of security and integrity for the controlled environment, ensuring no contamination.
By implementing our modular air supply systems, you can safeguard product integrity, protect operators, and ensure compliance with current Good Manufacturing Practices (cGMP).

Quality
BECARV upholds internal policies and procedures that guide our daily operations.
We take pride in presenting our ISO-9001:2015 certification, which emphasizes our commitment to excellence and precision across all our services. These accreditation reflect our continuous dedication to meeting the highest industry standards, ensuring the quality and reliability of our work.
Quality
policy

Certified
ISO-9001 :2015
Why Work with Becarv
Partnering with Becarv means you’ll benefit from expert advice and the latest technology.
Quality and Reliability
Becarv designs and manufactures equipment and customized solutions to the highest quality standards. With over 10 years of industry recognition, we are trusted for delivering reliable, aseptic solutions that minimize contamination risks and ensure the integrity of biopharmaceutical processes.
Operational Efficiency
By incorporating advanced automation, digitalization, and process control technologies, Becarv reduces manual intervention, boosts precision, and enhances reproducibility. These improvements lower risks in critical operations and increase overall efficiency by closing process steps.
Problem Solving
Becarv excels in designing specific equipment solutions that meet client needs. They integrates smoothly into existing processes, providing automated operation, data logging, and ergonomic optimization to solve unique challenges.
Book a demo
"*" indicates required fields





